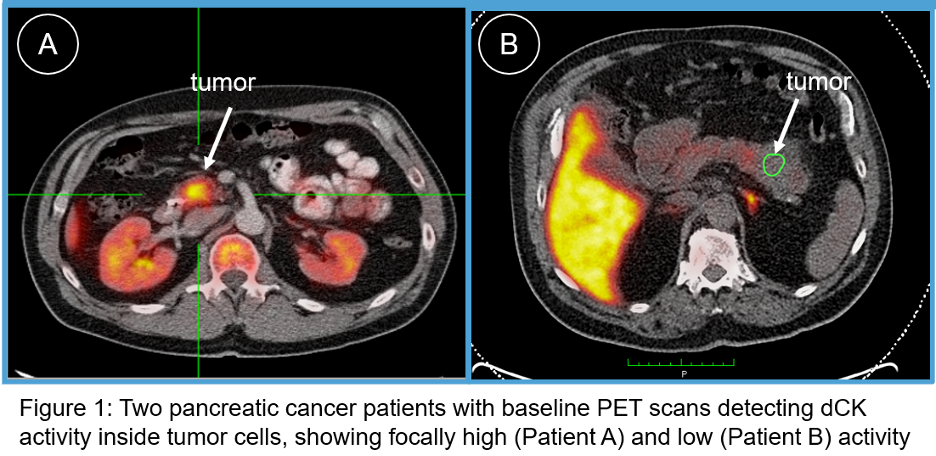
Trethera Announces Presentation at the Rare Neuroimmune Disorders Symposium Annual Meeting
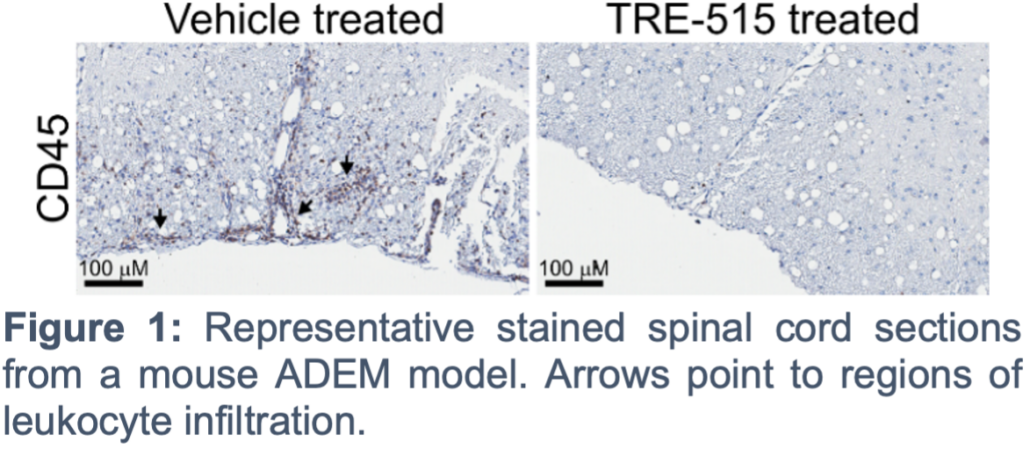
Los Angeles, October 15, 2024 — Trethera Corporation (“Trethera”), a clinical stage biopharmaceutical company committed to developing novel drugs targeting nucleotide metabolism for the treatment of cancer and autoimmune diseases, announces an upcoming presentation at the 7th Annual Meeting of the Rare Neuroimmune Disorders Symposium (RNDS). Trethera’s CEO, Dr. Ken Schultz, will present research highlighting the use of Trethera’s deoxycytidine kinase (dCK) inhibitor, TRE-515, as a treatment for optic neuritis and acute disseminated encephalomyelitis (ADEM).
The RNDS meeting is a specialized gathering of patients and translational science experts highlighting notable, novel and rigorous scientific discoveries made in rare neuroimmune disorders that advances understanding of the clinical care of patients with these diseases. Dr. Schultz’s presentation focuses on Trethera advancements in the treatment of optic neuritis, a rare neurologic disease which affects the optic nerve causing sudden blindness or visual impairment. The presentation also emphasizes ADEM, a severe autoimmune disease occurring mainly in 6 to 8 year-old children that can lead to loss of consciousness, coma, and death.
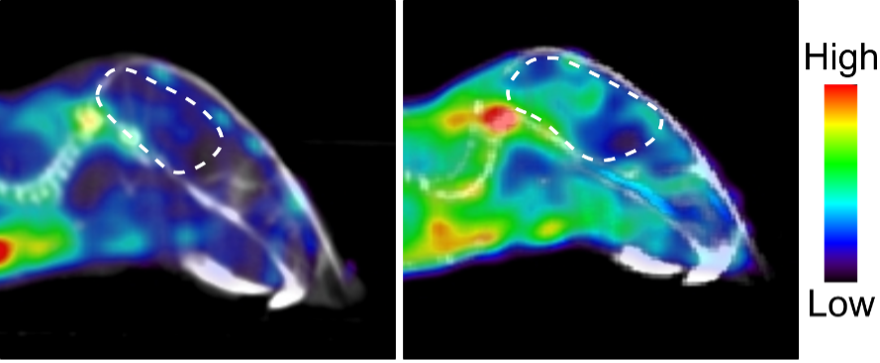
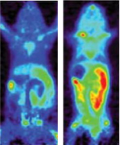
Figure 1: scans showing mouse brain before (left) and after (right) disease induction (brain encircled in a dashed white line). The enzyme dCK becomes upregulated during disease.
Trethera’s clinical stage and first-in-class drug, TRE-515, holds FDA Orphan Drug status for both optic neuritis and ADEM. FDA Orphan Drug designation confers substantial advantages, including a faster path to market approval, FDA assistance in designing clinical trials, exemption from the $4M drug approval application fee, and eligibility for seven years of marketing exclusivity. Should the FDA approve TRE-515 for commercial use in ADEM, Trethera would be eligible for a pediatric priority review voucher. TRE-515 is currently being evaluated in a Phase 1 dose escalation solid tumors clinical trial.
Presentation Details:
- Presentation Title: Advancements in ADEM & optic neuritis treatment with TRE-515: A clinical stage, dual orphan drug designated, first-in-class, deoxycytidine kinase inhibitor
- Presenter: Kenneth A. Schultz, MD
- Session: Research on Rare Neuroimmune Disorders, Part I
- Date: Saturday, October 19, 2024; 9:00AM – 11:15AM
- Location: Main Ballroom, Hyatt Regency; 2334 N International Pkwy, Dallas, Texas
About Trethera
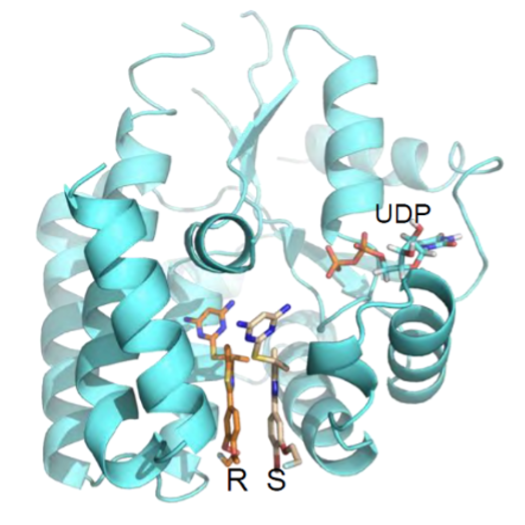
Trethera is a clinical stage, privately held, biopharmaceutical company dedicated to pioneering the development of novel treatments for autoimmune diseases and cancers. Founded by prominent UCLA scientists, Trethera is led by experienced management and board members. Trethera’s innovative approach to targeting nucleotide metabolism led to the development of TRE-515, an orally administered capsule twice designated by the FDA as an Orphan Drug. TRE-515 is a first-in-class clinical stage drug that inhibits deoxycytidine kinase (dCK), the rate-limiting enzyme in the nucleoside salvage pathway, one of two biosynthetic pathways that generate DNA precursors. It is believed that some forms of cancer may be preferentially dependent on the salvage pathway to support tumor growth, and certain autoimmune diseases might also respond to TRE-515 treatment. Trethera is developing TRE-515 for use as a monotherapy or in combination to precisely target a metabolic vulnerability of cancer or autoimmune diseases that will transform outcomes for patients.
For more information, please visit us at trethera.com or e-mail Investor Relations at ir@trethera.com.
Note on Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that Trethera believes or anticipates will or may occur in the future are “forward-looking statements,” which may often, but not always, be identified by the use of such words as “may,” “might,” “will,” “will likely result,” “would,” “should,” “estimate,” “plan,” “project,” “forecast,” “intend,” “expect,” “anticipate,” “believe,” “seek,” “continue,” “target” or the negative of such terms or other similar expressions. Although Trethera has a reasonable basis for the forward-looking statements contained herein, Trethera cautions that such statements are based on current expectations about future events and are subject to risks, uncertainties and factors relating to medical and scientific research, all of which are difficult to predict and many of which are beyond Trethera’s control, that may cause actual results to differ materially from those expressed or implied by the forward-looking statements in this press release. These potential risks and uncertainties include, without limitation: the extent to which development of any novel cancer therapies or therapies for autoimmune diseases succeeds; whether Trethera would obtain the necessary regulatory approvals to commence human trials or commercialize TRE-515 or any novel therapies resulting from such research; Trethera successfully implementing its growth strategy, including that relating to its disease therapies; the effects of the global Covid-19 pandemic; changes in economic conditions; competition; and risks and uncertainties applicable to the business of Trethera. The statements in this press release speak only as of the date hereof and Trethera does not undertake any obligation to update, amend or clarify these forward-looking statements whether as a result of new information, future events or otherwise. The Company intends that all forward-looking statements be subject to the safe-harbor provisions of the Private Securities Litigation Reform Act of 1995.